Key Points
- Chinese scientists led by Wang Chunyang (王春阳) from the Chinese Academy of Sciences have, for the first time, directly observed the solid-state battery short circuit mechanism at the nanoscale using in-situ transmission electron microscopy (TEM).
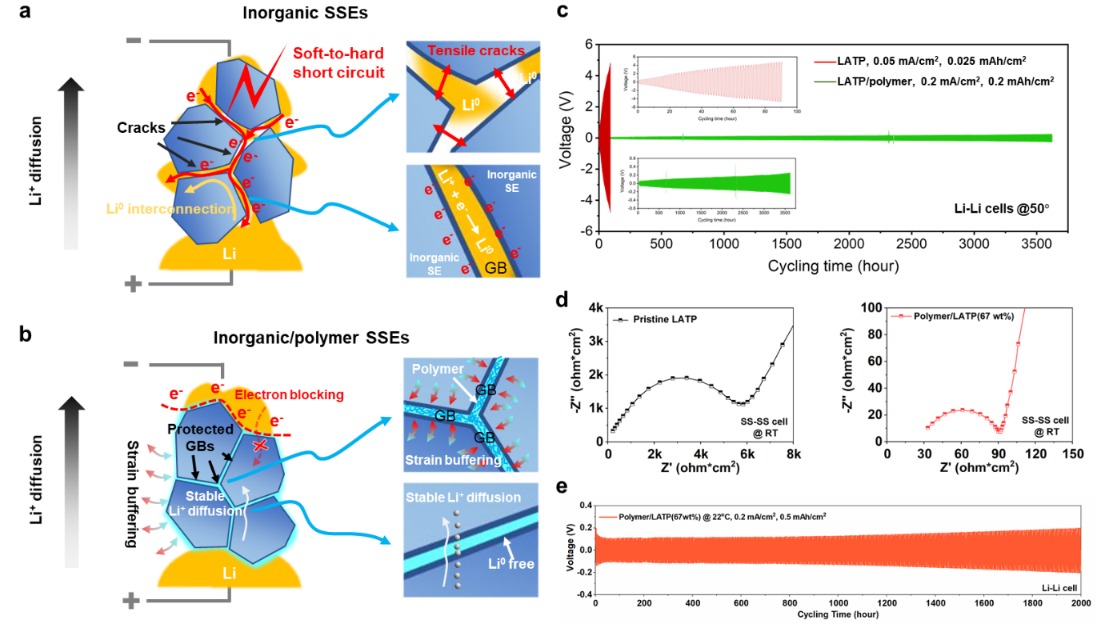
- The study reveals that short circuits in solid-state batteries occur in a two-stage process: a gradual “soft shorting” evolving into irreversible “hard shorting” due to lithium metal precipitation along defects.
- This failure mechanism is identified as universal in common inorganic solid electrolytes like NASICON-type and Garnet-type.
- Based on their findings, the team developed inorganic/organic composite solid electrolytes with a protective 3D polymer network that successfully suppressed short-circuit failure.
- Published in the Journal of the American Chemical Society (《美国化学会会刊》), this research provides crucial insights for designing more reliable and safer next-generation solid-state batteries.

Ever wondered what powers your smartphone or that sleek electric vehicle (EV) you’ve been eyeing?
Chances are, it’s a lithium-ion battery.
But here’s the catch: traditional liquid lithium-ion batteries, while common, come with certain safety risks.
That’s why the tech world is buzzing about “all-solid-state batteries.”
These next-gen powerhouses swap out leaky liquid electrolytes for solid ones.
The dream? Safer batteries, potentially with even higher energy density, especially when paired with lithium metal anodes.
But there’s a stubborn gremlin in the works: these advanced batteries can unexpectedly short circuit and fail.
Understanding why this happens is key to unlocking their full potential, especially for the solid-state battery short circuit mechanism.

Find Top Talent on China's Leading Networks
- Post Across China's Job Sites from $299 / role
- Qualified Applicant Bundles
- One Central Candidate Hub
Your First Job Post Use Checkout Code 'Fresh20'
The Big Breakthrough: Peeking Inside Solid-State Batteries
Here’s some exciting news that could change the game.
A team led by Researcher Wang Chunyang (王春阳) from the Shenyang National Research Center for Materials Science, Institute of Metal Research, Chinese Academy of Sciences (中国科学院金属研究所沈阳材料科学国家研究中心), has made a significant leap.
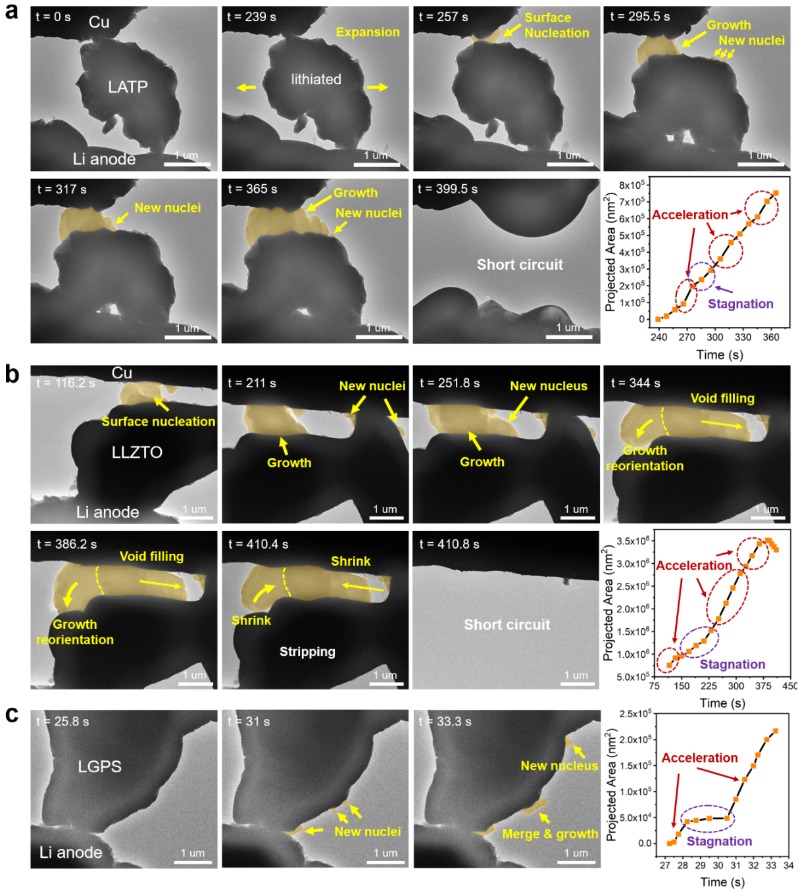
Working with international collaborators, they’ve, for the first time ever, actually seen how these short circuits happen at the nanoscale.
How? By using a super-powerful technique called in-situ transmission electron microscopy (TEM).
This allowed them to observe the soft-short to hard-short transition mechanism in inorganic solid electrolytes and the lithium deposition kinetics behind it.
These groundbreaking findings were published in the prestigious Journal of the American Chemical Society (《美国化学会会刊》) on May 20th.

ExpatInvest China
Grow Your RMB in China:
- Invest Your RMB Locally
- Buy & Sell Online in CN¥
- No Lock-In Periods
- English Service & Data
- Start with Only ¥1,000

Resume Captain
Your AI Career Toolkit:
- AI Resume Optimization
- Custom Cover Letters
- LinkedIn Profile Boost
- Interview Question Prep
- Salary Negotiation Agent

Understanding the Enemy: How Solid-State Batteries Short Circuit
So, what did these in-situ TEM observations reveal about the solid-state battery short circuit mechanism?
It turns out that lithium metal building up (precipitation) and forming interconnected electronic pathways due to tiny internal flaws in the solid electrolyte—like grain boundaries and voids—are the direct culprits.
This failure doesn’t just happen all at once. It’s a two-stage process:
- Soft Shorting: The Sneaky Start
- Hard Shorting: The Point of No Return
Stage 1: The “Soft Short” – Like Roots in a Pipe
Soft shorting kicks things off.
Imagine tiny bits of lithium metal forming at the nanoscale.
This lithium grows along existing defects in the electrolyte, like tree roots spreading through cracks.
These “roots” create transient conductive pathways – temporary electrical leaks, if you will.
It’s a subtle problem at first, but it’s the beginning of the end.
Stage 2: The “Hard Short” – Game Over
As soft shorts happen more frequently, the short circuit current increases.
The solid electrolyte, almost like it’s being “trained,” starts to form more permanent, memory-like conductive channels.
Eventually, it completely loses its ability to insulate.
Boom: irreversible hard shorting.
Think of it like this: at tiny cracks inside the battery, nanoscale lithium metal “corrodes” the material structure, much like mercury seeping into and weakening other metals.
This causes brittle fractures to spread, taking the battery from a temporary leak (soft short) to a complete, permanent short circuit (hard short).
The researchers also found that this failure mechanism isn’t a one-off; it’s a universal problem in common types of inorganic solid electrolytes, specifically NASICON-type and Garnet-type.
This is a huge insight for anyone working on solid electrolyte development.
- Two-stage process: Soft Shorting -> Hard Shorting
- Driven by Lithium metal precipitation along defects (grain boundaries, voids)
- Creates transient, then permanent conductive pathways
- Short circuit current increases from soft to hard phases
- Universal problem across NASICON-type and Garnet-type electrolytes
- Nanoscale lithium “corrosion” weakens structure, causing fractures


Finding a Fix: A New Approach to Stronger Electrolytes
Knowing why something breaks is the first step to fixing it.
And that’s exactly what this research team did.
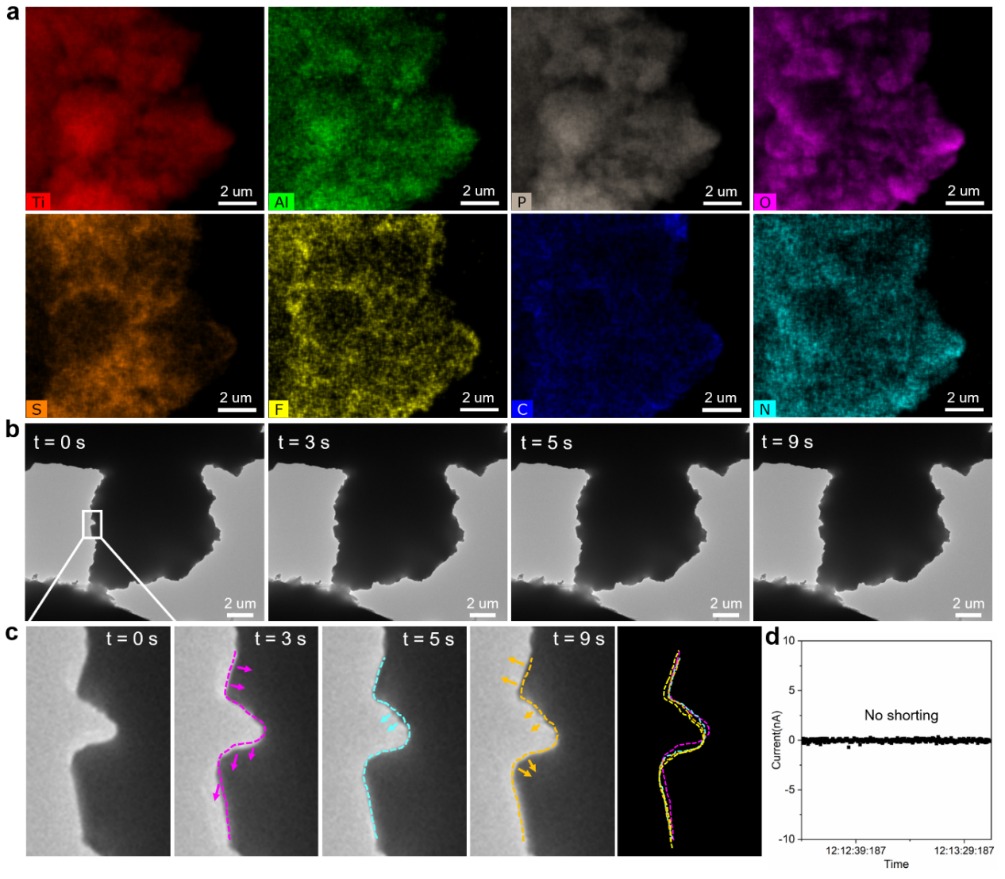
Based on their new understanding of the solid-state battery short circuit mechanism, they developed a clever solution: inorganic/organic composite solid electrolytes.
What’s special about them?
They feature a three-dimensional electronically insulating and mechanically elastic polymer network.
Sounds complex, but the result is simple and effective:
- It significantly suppressed lithium metal precipitation.
- It prevented the interconnection of these lithium structures.
- It stopped the induced short-circuit failure within the solid electrolyte.
The bottom line? This new composite material dramatically enhanced the electrochemical stability of the solid electrolyte.
This is a big win for creating more reliable and safer solid-state batteries.

Why This Matters: Paving the Way for Better Batteries
So, what’s the big takeaway for investors, founders, techies, and marketers?
This research isn’t just an academic exercise.
By clearly showing the soft-short to hard-short transition mechanism in solid electrolytes and its direct link to how lithium deposits, this study offers brand-new insights into why these promising batteries can fail at the tiniest scale.
More importantly, it provides a strong theoretical basis for designing and developing even better, next-generation novel solid electrolytes.
Think longer-lasting, safer batteries for everything from your next smartphone to the electric vehicles that will dominate future roads.
Understanding the intricacies of the solid-state battery short circuit mechanism is a critical step towards that future, and this work brings us significantly closer.

References
- CCTV News
- Tracking the Soft-to-Hard Short Transition in Inorganic Solid Electrolytes by In Situ Transmission Electron Microscopy – Journal of the American Chemical Society
- Suppressing dendrite formation in solid-state batteries – Nature Energy
- Interphase design for lithium metal solid state batteries – Science




